
The investigator should have a plan for how the study will be supervised. While an investigator may delegate to appropriately qualified and trained individuals tasks associated with evaluating and assessing adverse events, the investigator is responsible for ensuring these tasks are appropriately carried out. The investigator is accountable for regulatory violations resulting from failure to adequately supervise the conduct of the clinical study.
#When evaluating the causality of an adverse event trial#
The investigator should have sufficient time to properly conduct and supervise the clinical trial, and the level of supervision should be appropriate to the staff, the nature of the trial and the subject population. When tasks are delegated by an investigator, the investigator is responsible for providing adequate supervision of those to whom tasks are delegated. While it is common practice for investigators to delegate certain study-related tasks to employees, colleagues or other third parties (individuals or entities not under the direct supervision of the investigator), the investigator remains responsible for all study-related tasks. This would include documenting and reporting adverse events. However, after reading multiple documents/guidances, it seems that the assessor could be a registered nurse as long as that person is qualified, licensed and delegated to do so by the principal investigator.Īnswer: FDA regulations hold the investigator responsible for: ensuring that a clinical investigation is conducted according to the signed investigator statement (for clinical investigations of drugs, including biological products) or investigator agreement (for clinical investigations of medical devices), as well as the investigational plan and applicable regulations for protecting the rights, safety and welfare of subjects under the investigator’s care and for controlling drugs, biological products and devices under investigation. Question: Who can assess relationship/causality for adverse events? When starting out in this position, I was told by superiors that it must be a medical doctor or doctor of osteopathy.

The following is a selection of questions received and answered by GCPP officials. Examples of common drug-induced reactions in these organ systems are also provided to demonstrate the process of assessing such reactions.The FDA’s Good Clinical Practice Program (GCPP) responds to inquiries on a variety of trial-related subjects, providing answers on the agency’s official regulations as well as best practices. In addition to an introduction to the methodology for evaluating causality, this chapter focuses on the evaluation of side effects seen in commonly affected organs/organ systems (the liver, kidney, skin, and cardiovascular system) and highlights the factors that may need to be considered within these areas to determine a causal relationship.
+inference+on+the+grade+of+the+relationship.jpg)
The ability to discern if a side effect is truly caused by a specific drug or is confounded by the patient’s concurrent medical condition(s) and/or concomitant medications is an art based on the totality of the available evidence combined with a systematic approach. Examples of common drug-induced reactions in these organ systems are also provided to demonstrate the process of assessing such reactions.ĪB - An adverse drug reaction is a side effect for which the cause can be directly attributed to a drug and its physiologic properties. N2 - An adverse drug reaction is a side effect for which the cause can be directly attributed to a drug and its physiologic properties.
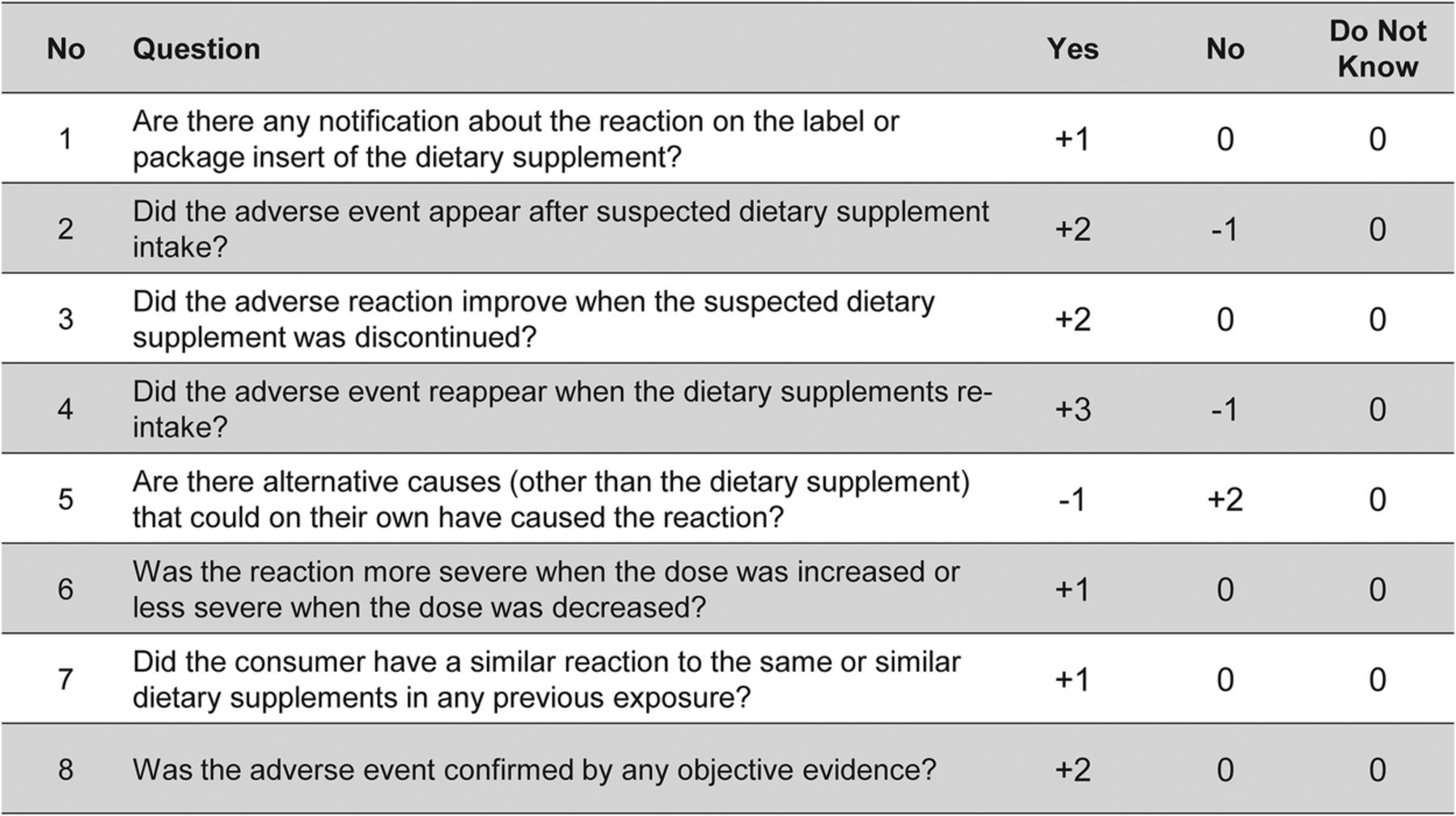
T1 - Causality assessment and examples of adverse drug reactions (drug-induced liver injury, renal, skin, and major adverse cardiac events)


 0 kommentar(er)
0 kommentar(er)
